LentiBOOST Transduction Enhancer
LentiBOOST®
LentiBOOST®
LentiBOOST®
Overview
Lentivirus Transduction Enhancer LentiBOOST®: the new Gold Standard for Clinical Applications
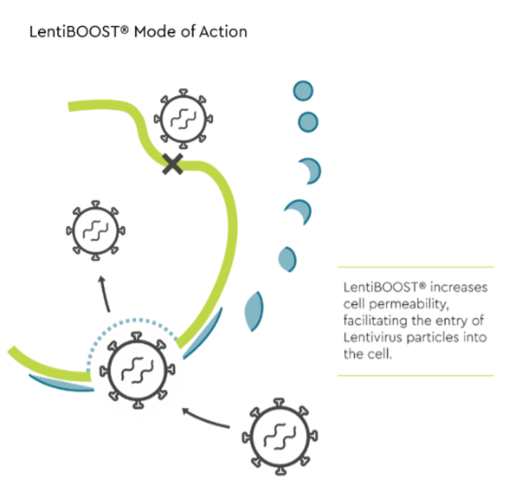
LentiBOOST Mode of Action
LentiBOOST is a highly effective, non-cytotoxic transduction enhancer for clinical application of lentiviral vectors. LentiBOOST acts as a universal receptor-independent adjuvant which facilitates fusion of lentivirus with the cell membrane, increases vector copy number, and significantly improves lentivirus transduction efficiency.
Effective in a Wide Range of Cell Types
LentiBOOST can be applied to a wide range of clinically relevant cell types, including CD34+ hematopoietic stem cells (HSC), mesenchymal stem cells (MSC), neuronal stem cells, primary T cells, hard-to-transduce murine T cells, NK cells, and fibroblasts. It is ideal for clinical transduction protocols for ex vivo gene therapies and CAR-T cell therapies.
Increase in Transduced Cells with LentiBOOST
| Cell Type | Cell Type Transduction Efficiency Ratio with LentiBOOST | Cell Type | Cell Type Transduction Efficiency Ratio with LentiBOOST |
| Human CD34 + HSC | 1.6-7x | Human Fibroblasts | 2x |
| Human CD + T cells | 1.6-3x | Human PBMC | 2-3x |
| Human CD4 + T cells | 1.5x | Human CD8+ T cells | 2-x |
| Human CD3 + T cells | 6.5x | Human CD4+ T cells | 2.7x |
| Human NK cells | 3x |
Unit-fold increase in transduced cells when using LentiBOOST, based on customer data. The range depends on the experimental setup, vector design, and transduction protocols.
Benefits for Drug Development
- Up to 90% improved lentiviral transduction efficiency: Increases the expression levels of therapeutic protein and success of clinical trials
- Increased and titratable vector copy number per cell: Increased safety in line with FDA/EMA criteria
- Easy-to-use: No need to change the existing transduction protocol
- Pharma and GMP grade batches: GMP grade with necessary documentation for clinical trials
|
Successful track record of integration into more than 35 Phase I/II and III clinical trials in the US, Europe, and China as well as manufacturing of an approved product.
|
Applications and Cell Types
HSC Transduction and Clinical Applications of LentiBOOST
Engineered hematopoietic stem cells (HSC) are a key tool for treating hereditary diseases by transplanting these cells to restore the function of a missing gene. Among the diseases currently assessed in preclinical and clinical studies are, for example (Hauber et al., 2018):
-
Wiskott-Aldrich syndrome (WAS)
-
X-linked severe combined immunodeficiency (SCID-X1)
-
Blood β-thalassaemia
-
Sickle cell anemia
-
Neurodegenerative storage disorders
-
Treatment or prevention of HIV
A crucial prerequisite for treating these diseases is to develop a safe and robust manufacturing process that allows reliable production of the cell therapeutic product.
Transduction of Hematopoietic Stem Cells (HSC)
Transducing primary CD34+ HSC can be challenging. However, a standardized process is crucial for achieving approval of clinical development and market authorization of a cellular therapeutic. In the majority of cases, lentiviral vectors are used for transduction of HSC. There have been several attempts to improve lentiviral gene transfer rates by adding different substances as transduction enhancers. SIRION’s proprietary transduction enhancer LentiBOOST has become the worldwide gold standard for transduction enhancement in HSC. In addition researchers observed a synergistic effect with Protamine sulfate (Schott et al., 2019)
Improved transduction and optimal copy number per cell
Using LentiBOOST our customers observe improved transduction of their HSC. This has been shown experimentally: The number of GFP-positive human CD 34+ PBSC transduced with lentivirus and LentiBOOST at various concentrations reached up to 80% at day 12 post transduction.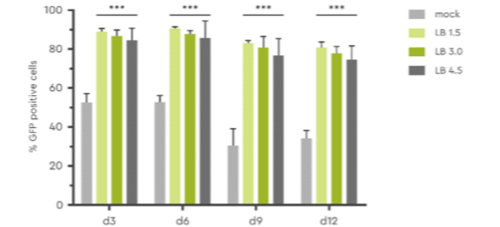
Figure 1: The amount of GFP positive human CD34+ PBSC was determined at day 3, 6, 9, and 12 post transduction at various concentrations of transduction enhancer (LB; 1.5 mg/ml to 4.5 mg/ml). Source: Hauber et al., 2018
A valuable feature of LentiBOOST is that vector copy number per cell is increased and can be titrated to optimum alignment with EMA/FDA safety guidelines to express the most suitable amount of your therapeutic protein. This can be achieved using various LentiBOOST concentrations.
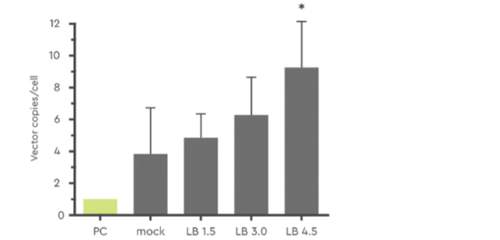
Figure 2: Effect of varying concentrations of LentiBOOST (1.5 to 4.5 mg/ml) on transduction efficiency with LV-GFP (MOI=10) in human CD34+ PBSC. The star (*) signifies p ≤ 0.05. Source: Hauber et al., 2018
Non-toxic and healthy HSC differentiation potential
Optimizing transduction efficiency must not be done at the expense of cell health or functionality. This has been assessed in various studies and HSC treated with LentiBOOST demonstrated the same viability as the control cells.
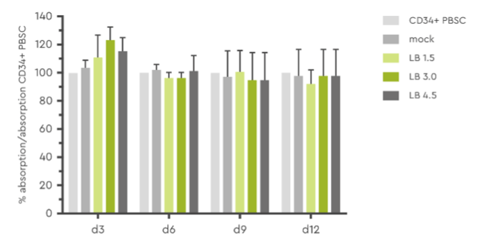
Figure 3: Determination of cell viability and toxicity in a MTT assay at day 3, 6, 9, and 12 post transduction. Source: Hauber et al., 2018
At least as important as health is the differentiation potential of the cells. Experiments have shown that LentiBOOST does not affect the ability of HSC to differentiate into various hematopoietic lineages.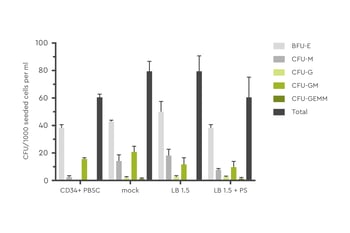
Figure 4: HSC differentiation potential was assessed by colony forming unit (CFU) assay. Cells were transduced in presence or absence of LentiBOOST (1.5 mg/ml) and protamine sulfate (5 µg/ml).Source: Hauber et al., 2018
Proven track record in clinical trials with HSC
Many academic and industrial organizations are using LentiBOOST in their clinical trials to improve their transduction protocols for hematopoietic stem cells (HSC). A selection of our LentiBOOST users in clinics:
-
Mustang Bio: X-linked severe combined immunodeficiency (NCT01306019)
Mustang Bio from the US licensed SIRION Biotech’s LentiBOOST technology in late 2020. They use our transduction enhancer in their MB-207 program that is assessing a lentiviral therapy for the treatment of patients with X-linked severe combined immunodeficiency. The current status (as of end of 2021) of clinical development is a Phase 1/2 study. More here
-
University of California San Francisco: Artemis-deficient Severe Combined Immunodeficiency (NCT03538899)
The goal of this UCSF study is to treat patients with Artemis-deficient Severe Combined Immunodeficiency who lack a donor for stem cell transplantation or who did not develop a functioning immune system after transplantation. As a treatment the patients receive their own stem cells that have been transduced with a self-inactivating lentiviral vector carrying a normal copy of the DCLRE1C gene to restore the function of their immune system. Currently this treatment is assessed in a Phase 1/2 study (as of end of 2021). More here
-
National Institute of Allergy and Infectious Diseases: X-linked severe combined immunodeficiency
The National Institute of Allergy and Infectious Diseases licensed our LentiBOOST technology to include in their program to treat patients with X-linked severe combined immunodeficiency (SCID-X1). Within that clinical trial the outcome for patients treated with engrafted CD34+ cells that have been transduced using a lentiviral vector is assessed. The cells are transduced to express a healthy version of the gene ILR2G that is impaired in patients suffering from SCID-X1. More here
-
Orchard Therapeutics: Rare Diseases
In late 2018 the commercial-stage biopharmaceutical company Orchard Therapeutics licensed SIRION Biotech’s LentiBOOST technology to include the transduction enhancer in its gene therapy products to treat patients with rare diseases. More here
T Cell Transduction and Clinical Applications of LentiBOOST
Engineered T cells are highly promising agents for therapeutic application in gene and cell therapies.
Therapeutic applications of engineered T cells
By far the most use for T cell therapies are in the oncological field (Sadelain et al., 2017). However, there are also other applications such as treatment of serious viral infections like HIV. Further indications are autoimmune diseases, graft-versus-host-disease as well as transplantation tolerance.
In oncology there are three major fields that leverage engineered T cells and have shown promising responses in patients suffering from cancer (Simon et al., 2019) :
-
Engineered T cells that express a tumor antigen-specific chimeric antigen receptor (CAR T cells)
-
T cells that were modified to express a tumor antigen-specific T cell receptor (TCR T cells)
-
Tumor-infiltrating lymphocytes (TILs)
Further development of CAR-T cell therapy: CAR-NK
Our LentiBOOST® technology is widely used in the CAR-T field. One potential application is a GMP compliant manufacturing process for allogeneic CD19-CAR-T cells described by translational researchers in the journal Gene Therapy (Kim-Hoehamer et al., 2022).
Due to the great clinical success of CAR-T therapies in recent years, a derived application is becoming more and more popular: CAR-NK therapies. According to the register clinicaltrials.gov the first CAR-NK therapeutics are in clinical trials and the first Biotech/Biopharma companies are building their business around technology platforms that include CAR-NK cells.
The advantages of such therapies could be (Xie et al., 2020) :
-
A safer cell therapy product
-
Multiple mechanisms mediating cytotoxicity
-
The possibility to develop an off-the-shelf product
T cell transduction: Including LentiBOOST in the manufacturing process
Generating sufficient numbers of T cells is an important prerequisite to developing a T cell therapy that can be broadly applied while ensuring that all patients can profit from the therapy (Simon et al., 2019). This is especially important for autologous applications, but allogeneic procedures also require a robust manufacturing process that ensures the product can be produced with consistent quality, while using resources efficiently.
Transduction enhancers such as our proprietary LentiBOOST technology optimize T cell manufacturing processes that do not yet deliver the desired results in terms of transduction efficiency or that need to improve their use of resources.
A SIRION Biotech customer made a head-to-head comparison of LentiBOOST with other transduction enhancers in human T cells. The cells were transduced with LentiBOOST and the other reagents at various concentrations. LentiBOOST showed the strongest effect at all three concentration levels. At the highest concentration the transduction enhancing effect of LentiBOOST was the strongest, with efficiency about 5-times more than transduction without an enhancer.
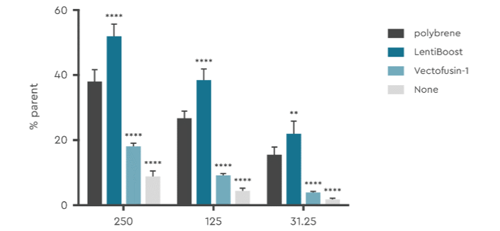
Figure 5: Customer Data
In their 2021 paper Presti and colleagues analyzed LentiBOOST use in the context of engineering CD8+ T cells for use in cancer therapy (lo Presti et al., 2021). They also saw that LentiBOOST strongly enhanced transduction efficiency. This transduction enhancing effect is more pronounced at a lower MOI - in this case equal to 1. The authors also looked at the viability of the cells after transduction with and without LentiBOOST. Here, the scientists observed only a minimal, non-significant reduction in viability. Presti and her colleagues were also able to demonstrate the transduction enhancing effect of LentiBOOST in a T cell transduction with a CAR construct (directed against CD19).
Use of LentiBOOST in clinical trials with engineered T cells
There are various LentiBOOST users active in the field of T cell therapies. A selection of licensees we are able to disclose:
-
Cellectis: Allogeneic CAR-T cell therapy
The biopharmaceutical company Cellectis licensed SIRION Biotech’s LentiBOOST technology in early 2021 to expand its portfolio of technologies for manufacturing its allogeneic CAR-T cells. Allogeneic CAR-T cell therapy represents a future-defining shift in simplicity, availability, and cost efficiency of immune-oncology approaches. More here
-
Beam Therapeutics: New generation of CAR-T products
In early 2020 Beam Therapeutics licensed our LentiBOOST technology for use in their CAR-T cell products. The US company is developing a new generation of CAR-T product candidates using its proprietary base editing technology.
Hard-to-transduce murine cells: in preclinical proof-of-concept studies
One potential cause of problems in preclinical experiments is that murine T cells and other murine cell types do not transduce as well with lentiviral vectors as human cells (Delville et al., 2018). However, the preclinical experiments are only transferable if they are performed with the lentiviral vector that will later be used in the clinic. LentiBOOST is a helpful tool to increase transduction of murine cells with the lentiviral construct that could become later part of a clinical program.
A group of scientists around Marianne Delville transduced murine T-cells in presence of LentiBOOST at different concentrations and compared the enhancing effect with the effect of protamine sulfate (Delville et al., 2018.) The researchers observed a strong enhancement effect by LentiBOOST® and a weaker effect in presence of protamine sulfate. This effect increased with a higher concentration of LentiBOOST was even stronger at day 12 post transduction compared to day 5. The authors could show this effect in murine hematopoietic stem cells as well.
Supply
LentiBOOST is available globally in pharma grade for research and process development purposes as well as GMP grade for clinical use. The GMP grade material is only available under a clinical license from SIRION Biotech. Both LentiBOOST grades are shipped worldwide.
To make the ordering and delivery process for our global customers as easy and fast as possible we also work with local Agents and Distributors to supply pharma grade material. GMP grade material can be obtained exclusively via SIRION Biotech.
In these countries LentiBOOST pharma grade can be ordered directly from us or from our trusted partners:
-
USA, Canada: Mayflower Bioscience
-
China: MineBio Life Sciences Ltd.
-
South Korea: KOMABIOTECH
-
Japan: BizCom Japan, Inc.
-
Rest of the world: Order LentiBOOST directly from SIRION Biotech.
LentiBOOST Pharma grade for Research Use Only
SIRION supplies LentiBOOST pharma grade (non-GMP) material for research and process development including large-scale runs. LentiBOOST pharma grade is used in hundreds of laboratories worldwide.

How much LentiBOOST do I need?
Material is supplied at a concentration of 100mg/ml.
For most applications, LentiBOOST is applied at a dilution of 1:100 to 1:400.
LentiBOOST GMP Grade for Clinical Use
LentiBOOST GMP grade material is available for clinical development and for cell therapies at the commercial stage under a clinical or commercial license. We know how important a reliable and secure supply chain is, especially in the commercial phase. We fulfill this responsibility to our business partners through our automated fill and finish process under GMP conditions in accordance with regulatory requirements. Our sophisticated system for quality assurance ensures compliance with highest standards and batch-to-batch consistency. LentiBOOST GMP grade comes with a CoA and all necessary documentation for regulatory authorities such as the FDA and EMA.
In 2023, SIRION will expand its product range to include LentiBOOST GMP-grade filled in 2R (1 mL) and 20R glass vials (12 mL). For more information reach out to our LentiBOOST team.
LentiBOOST Order Form
You can order directly here
Licensing
We want to help advance your clinical success by offering various licensing models to fit your needs. Join the growing community that relies on LentiBOOST for their lentiviral cell therapy programs
LentiBOOST Patent & Licensing Options
SIRION controls various patents* for the underlying substance class of LentiBOOST and licenses the technology for clinical use. All of our licensees profit from a secure IP position and avoid legal risks to their clinical programs.
We develop an individual licensing model that is aligned with your needs. Basically, SIRION Biotech offers two types of licenses: Academic licenses for investigator-led clinical trials and commercial licenses for companies that are developing and commercializing cell therapy programs.
Contact us now at licensing@sirion-biotech.de to ensure that you don't lose valuable time on your journey towards clinical approval.
*Intellectual property controlled by SIRION related to the underlying substance class of LentiBOOST: WO2013127964A1; EP2820138B1; EP3763820B1; EP 21163635.2; IL265014B; IL234331B; JP6290106B2; JP6638004B2; US 10,815,498B2; US 16/941,780; US 9,771,599B2; HK40044767; HK42022048344.0
Academic license for supply of LentiBOOST GMP
We want to support pioneers developing new cell therapies. LentiBOOST transduction enhancer helps clinical scientists generate better data, which will ultimately lead to better curative treatment options for patients. That’s why we are constantly working to make our LentiBOOST technology easily accessible for academic partners to use in their clinical programs.
SIRION Biotech grants an academic license provided that the non-profit research organization is responsible to the regulatory authorities for the clinical trial.
European as well as US research hospitals already profit from our academic license.
A selection of our academic partners:
Clinical/Commercial license for supply of LentiBOOST GMP
We work very closely with our licensees as a partner on the road to clinical approval of their cell therapy. LentiBOOST increases the response rate and overall success of clinical trials. In addition, licensees benefit from a secured IP position. By integrating LentiBOOST into their processes, our licensees make their manufacturing more robust and have access to GMP material for both clinical and commercial use.
A selection of our industry partners:
-
Beam Therapeutics
Resources
Product Manual: LentiBOOST Pharma Grade
LentiBOOST transduction enhancer can easily be integrated into standard transduction protocols. Our Manual can provide you with help working with LentiBOOST.
Product Flyer: Application of LentiBOOST Technology
This flyer details the benefit of using LentiBOOST® for lentiviral transduction and clinical applications, inlcuding scientific data on transduction efficiency and VCN in stem cells and T cells in the presence of LentiBOOST.
