Viral Vector Overview
Viral Vector Overview
Viral vectors are the most efficient vehicles for gene delivery into a specific cell type or tissue for a wide array of research purposes.
Regardless of the field of application, any viral vector therapy goes through a discovery phase where proof-of-concept is established, vectors are optimized, and safety and efficiency studies of the drug candidate are completed.
SIRION Biotech offers a comprehensive viral vector portfolio to professionals working in early discovery and preclinical research stages.
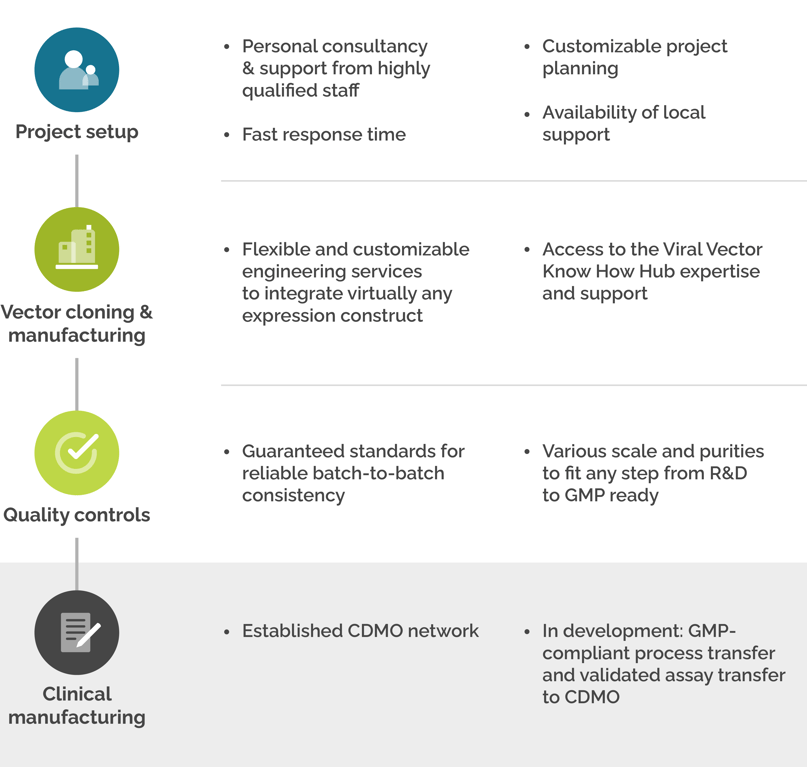
How SIRION Biotech can Support You
Working with SIRION has never been easier with individual customized solutions to fit any type of project in any stage of development.
Common Uses of Viral Vectors
Recombinant viral vectors have been modified and re-designed to take advantage of the virus’s specialized mechanism to introduce genetic material into host cells, without causing the diseases associated with the wild type virus.
Due to their individual biological characteristic, each type of viral vector is suited for specific applications.
Adeno-Associated viral vectors (AAV)
AAV is the leading platform for in vivo gene delivery for treatment of various human genetic disorders.
It was first discovered in 1965 as a contaminant of AV preparation and is one of the smallest viruses, belonging to Parvoviridae family. AAV is a non-enveloped virus containing a single-stranded 4.7 kb DNA genome which is packed into an icosahedral capsid of around 25 nm.
AAVs belong to the genus Dependoparvovirus as they replicate only in the presence of helper viruses such as adenovirus or herpes virus. However, techniques were developed for manufacturing of viral particles without the need for separation from the helper virus, which are now widely used.
AAV-based viral vectors are one of the safest and most common tools for clinical applications due to their relatively low immunogenicity and lower risk for insertional mutagenesis due to their ability to mediate long-term expression from episomal, non-integrated vector expression cassettes. Another safety feature of AAV-based vectors is that they do carry viral genes and cannot replicate in the target cells.
Drug development and preclinical studies
AAV vectors have excelled at in vivo disease modeling due to their long-term gene expression. Persistence of the AAV in its episomal state depends on the cell turnover in the targeted tissue and the number of AAV genomes in the cell. Persistence of AAV episomes over 1.5 years have been reported in tissues with dividing cells, and potentially indefinitely in post-mitotic tissues.
Within the drug developmental phase AAV vectors are used for pharmacological profiling and mechanism of action (PoC) studies. Pre-clinical studies may use AAV for:
- Animal pharmacology (biodistribution)
- Dosing and administration route determination
- Safety profiling (IND-Enabling Toxicology Studies)
For more detailed information about SIRION services for AAV vector design and manufacture, go to Viral Vector Design and Manufacturing/AAV.
In vivo gene therapy
The success story of AAV-based vectors has already lead to the clinical approval of patient life-changing drugs, such as Zolgensma treating the fatal genetic disease spinal muscular atrophy. There are currently more than 300 clinical trials based on AAV vectors addressing indications such as:
- Muscle diseases such as Duchenne Muscular Dystrophy, Limb Girdle muscular dystrophy, X-linked Myotubular Myopathy
- Neurologic disorders such as Parkinson’s Disease, Alzheimer's Disease, Dementia, Huntington’s disease
- Metabolic diseases such as Batten disease, Sanfilippo syndrome B, Crigler-Najjar Syndrome)
- Ophthalmic diseases such as Leber´s Hereditary Optic Neuropathies, Leber´s congenital amaurosis, Age-Related Maculophathies, Achromatopsia
For more detailed information about how SIRION can support developing AAV vector for gene therapy applications, go to AAV Vectors for Gene Therapy.
Current trends
The characterization and alleviation of AAV vector interaction with the immune system continue to be a major priority of the field, including vector design for reduced immunogenicity, and clinical management of anti-capsid and -transgene immunity. To allow for more targeted payload delivery with reduced vector dose, various AAV capsid engineering methods have been established, including computational, directed evolution and chemical modification methods.
Due to the risk of insertion mutagenesis associated with LV vectors and increased efforts in improving the safety of gene therapy, AAV is being investigated as an alternative tool for ex vivo modification of T Cells for autologous cell transplantation.
In 2016, transduction of primary human CD8+ T cells and targeted gene knock-in by homology-directed repair genome editing was achieved using AAV serotype 6 for donor delivery. Recently an in vivo CAR T cell strategy was tested by intravenous dosing the AAV-DJ variant.
These novel approaches using AAV-mediated in vivo gene delivery to immune cells could change the immunotherapy paradigm.
Lentiviral Vectors (LV)
Lentiviruses are a subtype of retroviruses which have a single stranded RNA genome that encode for three major structural genes: gag, pol, and env.
The intensive research on HIV in pursuit of a treatment for AIDS led to the development of recombinant HIV-based vectors. Lentiviral vectors contain only the viral cis-regulatory elements necessary for genome integration, transcription, and packaging of the vector RNA. All viral protein coding regions are removed providing space for insertion of a foreign DNA sequence. Separating the lentiviral genome onto several plasmids reduced the risk of generating replication competent lentiviruses
In contrast with classical retroviruses, lentiviral vectors have the ability to transduce both dividing and non-dividing cells which are used as general tools for basic research and translational applications.
A milestone was reached in 2017 when the first CAR-T LV-based therapy was approved by the FDA for treatment of refractory or relapse (R/R) B cell precursor acute lymphoblastic leukemia.
Currently there are more than 200 clinical trials ongoing worldwide using LV as a genetic delivery tool addressing a range of diseases.
Cell model development
LV are widely used for generation of cell lines that stably over-express genes of interest or hRNA/shmiR for knock-down approaches.
The resulting modified mammalian cell lines are most commonly used for:
- functional gene analysis
- drug screening assays
- antibody and protein expression
- study of cellular events such as cell differentiation
- assay development
- toxicity studies.
Ex vivo gene therapy (cell therapy)
The ability of LV to stably integrate into host genome for long-term gene overexpression made it a promising tool for treatment of monogenetic disease, hematological malignancies and solid tumors.
LV’s most common usage is in adoptive cell transfer therapies where it allows developers to deliver a therapeutic transgene (e.g. CAR or T cell receptors) into the desired cells. One way to classify ex vivo cell therapy is based on the cell type to be modified:
- T cells (CAR or TCR)
- Stem Cell
- TILs
- NK & NKT
- Tregs
- Macrophages
- Dendritic cells
For more detailed information about SIRION services for lentiviral vector design and manufacture, go to Viral Vector Design and Manufacturing/LV.
SIRION also offers LentiBOOST, our proprietary transduction enhancer providing up to 90% improved lentiviral transduction efficiency across a wide range of cell types. For more information please visit our LentiBOOST page here.
In vivo gene therapy
Pseudotyping LV with the VSV-G envelope proteins confers it with a wider tissue tropism and more efficient transduction capacity for a variety of cell types. Through direct injection or systemic delivery, LV can be used for gene expression in different organs:
- Liver (in different species: human, NHPs or dogs)
- Eye (corneal cells in mice or pigs)
- Lungs (mice)
- Skin tissue (major cell types, including fibroblasts, keratinocytes or endothelial cells)
- Brain (oligodendrocytes, Schwann cells, neurons)
For more detailed information about how SIRION can support developing lentiviral vectors for cell and gene therapy applications, go to Lentiviral Vectors for Gene Therapy.
Adenoviral Vectors (AV)
Adenovirus is a non-enveloped virus with a double-stranded linear DNA genome enclosed in an icosahedral protein shell.
It belongs to the family Adenoviridae and to date there are 88 accepted human adenovirus serotypes (HAdVs 1 to 88) which are sub-classified into seven species (A to G). Each serotype is known to cause different conditions such as infections of the upper respiratory tract, conjunctivitis, gastroenteritis, or pneumonia.
The adenoviral vectors most commonly used for gene therapies or vaccination are based on the Ad5 serotype, which has a large packaging capacity and is non-toxic and non-integrating. There is a high prevalence of preexisting anti-Ad5 immunity resulting from natural infection. The attachment and cell entry is mediated via Ad5’s fiber protein interaction with host cell coxsackie virus-adenovirus receptor (CAR).
To improve safety, SIRION’s recombinant AV vectors lack two essential genes, E1 and E3, found in the wild type virus. Deletion of E1 from the viral vector genome prevents the generation of replication-competent AV vectors so AV vectors cannot replicate in target cells. E1 for viral production is presented by the virus packaging cells (HEK293). E3 is not essential for virus production.
Vaccine applications
AV has the ability to induce strong and sustained innate and adaptive immune responses, in particular T cell-mediated immunity. This makes AV an ideal candidate vectors for developing vaccines against:
- Infectious diseases (SARS-CoV-2, HIV/SIV; Influenza; Ebola; Tuberculosis; Malaria, MERS etc.)
- Oncolytic AV3 for cancer treatment (head and neck cancer, breast cancer and colon cancer)
Research and development
AV vectors exhibit a broad host and tissue tropism with high infectivity, being widely used for transgene expression or knockdown approaches in both in vitro applications and in vivo animal studies.
Gene therapy
Due to its unique biological features, AV are used in ongoing gene therapy clinical trials for treatment of diseases such as carotid salivary dysfunction, varicose ulcer, macular degeneration, and myocardial infarction.
