Adenovirus
Custom Adenovirus Vectors for Discovery and Preclinical Applications
Custom Adenovirus Vectors for Discovery and Preclinical Applications
Custom Adenovirus Vectors for Discovery and Preclinical Applications
Adenoviral vectors offer high levels of transgene expression in broad cell types, including dividing and non-dividing cells. Adenoviral vectors are the method of choice whenever fast and transient gene expression for a very large gene or several genes is required, since the expression cassette has a total capacity of 7.5 kb. This provides space for flexible vector design with multiple transgenes delivered in one vector.
Vaccine development is another popular application for adenoviral vectors. The ability of adenovirus to induce immune response in vivo makes it a great tool for vaccine studies.
General Adenovirus Characteristics
- Transient gene expression in vivo and in vitro
- No host genome integration
- Large packaging capacity up to 7.5 kb gives a space for multiple gene expression cassettes
- Reliable gene delivery into dividing and non-dividing cells with almost 100% efficiency
- Fast results - gene expression within 24 h
- Adenovirus remains epichromosomal and therefore does not interfere with other host genes
Adenoviral Vector Engineering
Adenovirus are non-toxic, non-integrating, non-enveloped viruses with a linear double-stranded DNA. Adenoviral vectors are based on the Ad5 serotype. To enter cells, Ad5-based vectors use the Coxsackie-Adenovirus Receptor (CAR). To increase packaging capacity in recombinant vectors, two genes are deleted (E1 and E3). E1 is provided by the packaging cells (HEK293) and E3 is not essential for virus production. Deletion of E1 from the viral vector prevents viral replication.
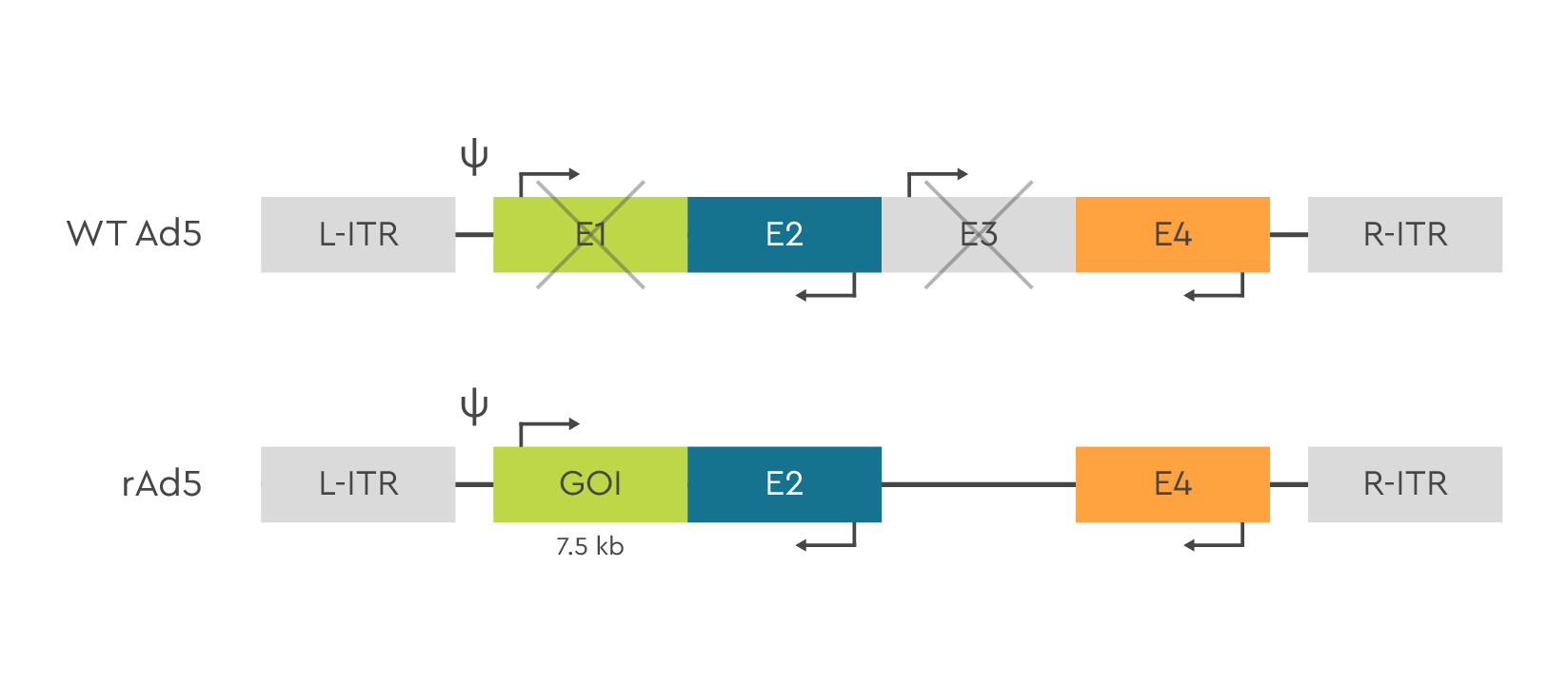
Figure 1. Schematic representation of the wild type Adenovirus vector genome organization and recombinant Ad5 vector. To generate the AV vector, E1 and E3 elements are removed, so a gene/genes of a total size of up to 7.5 kb can be inserted. E1 is provided with a packaging cell line HEK293.
Adenoviral Vector Manufacture
Production of adenoviral vectors is based on construction of an rAdV plasmid using the BAC system. The cDNA of your gene of interest (GOI)/shRNA is first cloned in an AV shuttle plasmid for either over expression (OEX) or knockdown (KD) followed by transformation into BA5-FRT cells containing the BAC vector containing the pAD5 vector genes. Recombination with the BAC vector is FLP-mediated. Pac 1 digestion removes the BAC-DNA and generates a linear Ad5-GOI construct.
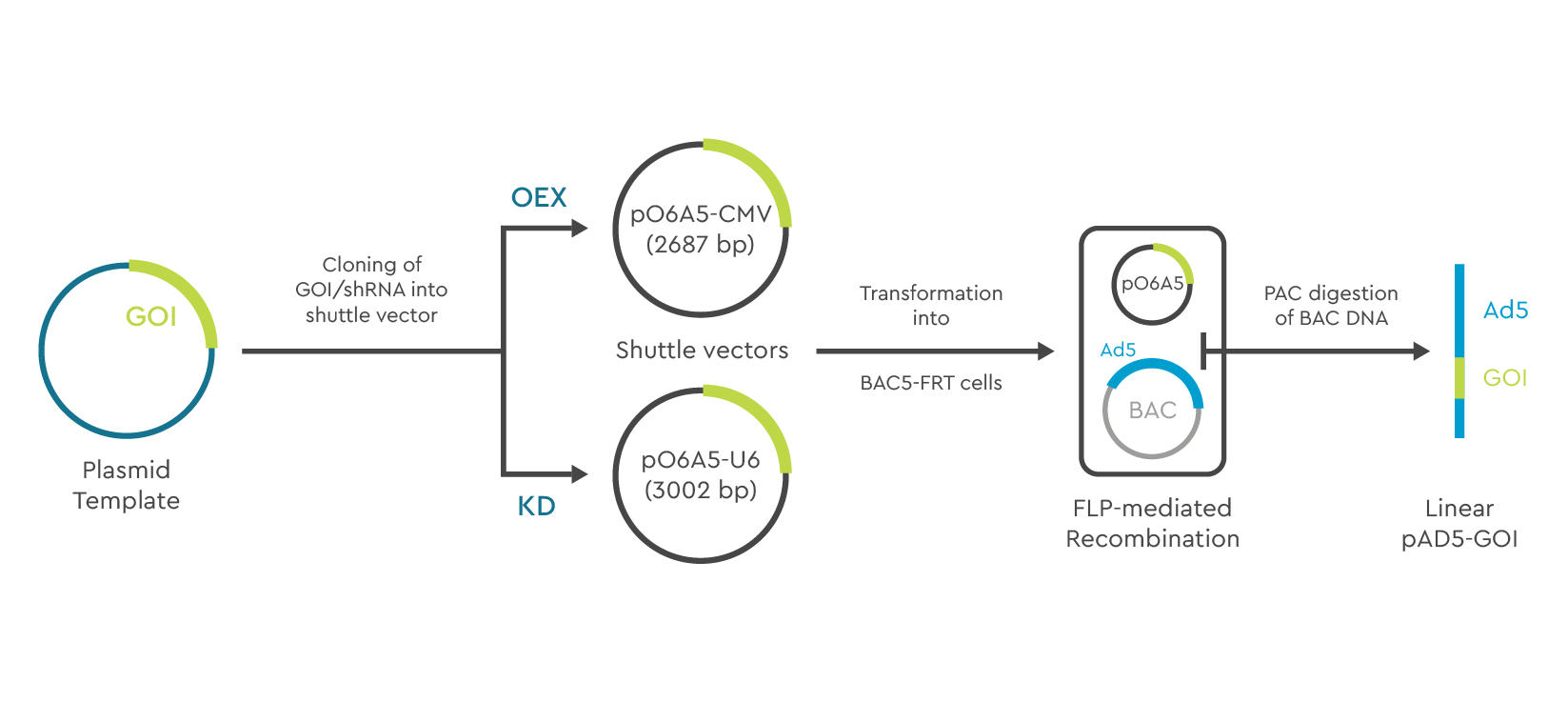
Figure 2. Generation of recombinant Adenoviral vectors using the BAC system
This linear construct is reconstituted into a recombinant adenoviral vector expressing the GOI by transfection into HEK 292 or CAP producer cells. This generates a primary low-titer virus stock. The viral titer is then amplified to the required scale, concentrated, and purified for QC testing. Adenoviral particles with GOI are then filled and finished ready for delivery.
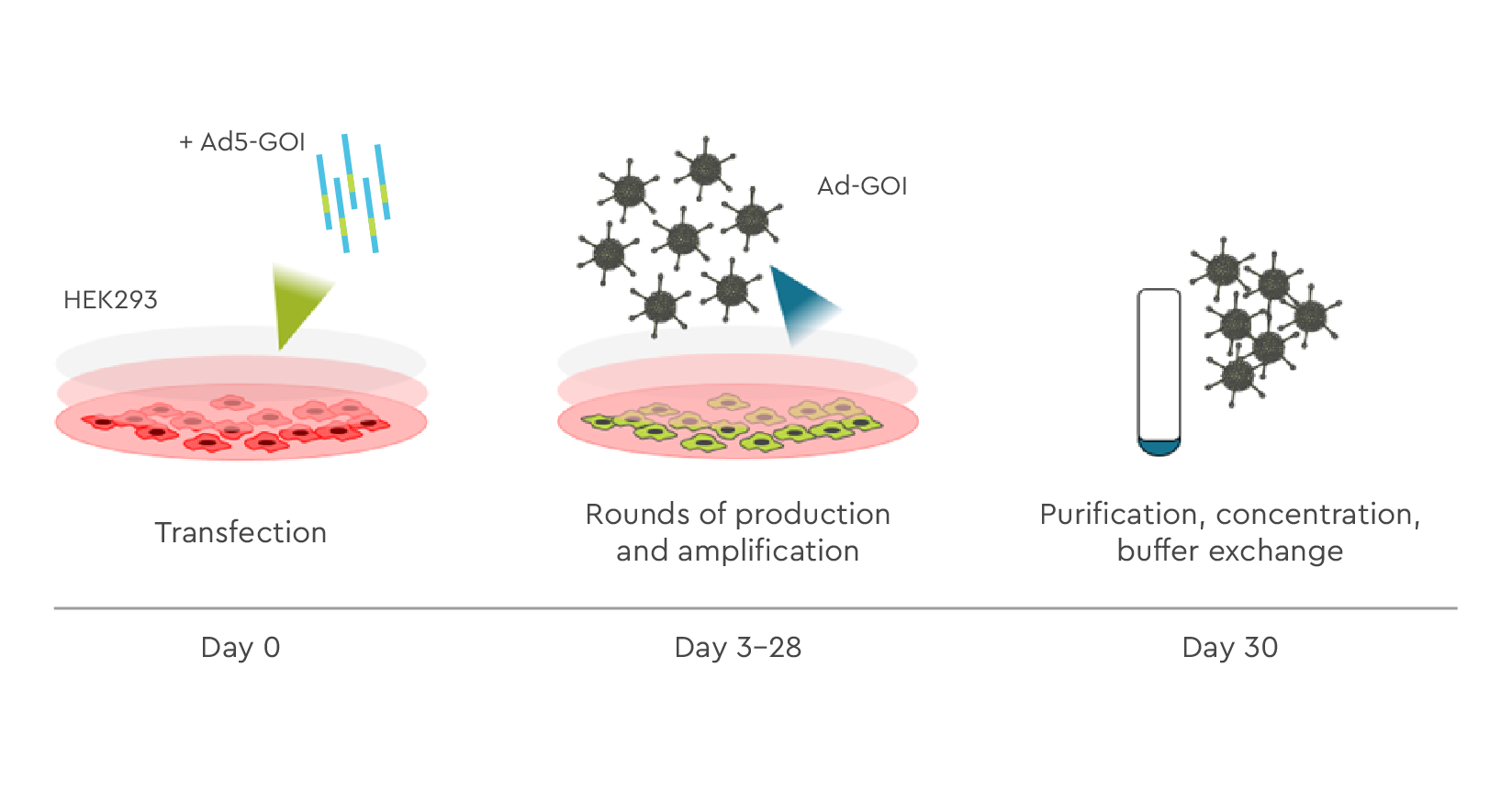
Figure 3. Schematic representation of Adenoviralvector production. At day 1 HEK293 cells are transfected with linear DNA containing Ad5-GOI. The cells are incubated for 2-3 days, then virus is collected from the supernatant and cell lysates. The virus collected from the first round is used for the infection of the cells in the 2nd round and for the larger amount of virus the 3rd round is used. The Ad-GOI is further purified and concentrated by CsCl (large-scale production) or using AdenoOne purification kit (small-scale productions).
QC and Analytics
SIRION offers various packages of Standard and Optional QC methods listed in Tables 1 and 2 below. Additional QC assays can be ordered separately. Please contact us directly if you would like more information.
Table 1. Standard Quality Control methods included in adenovirus services.
| STANDARD QC METHODS | |
| Infectious Units (IU) Titer | Determined via immunochemical detection of the adenoviral hexon protein in infected HEK293 cells. |
| Functionality Test in NIH3T3 Cells | NIH3T3 cells are transduced. Total RNA is isolated and 1 μg is reverse transcribed using a mixture of Random Hexamer and oligo-dT primer. Expression of the vector-encoded gene is determined by qRT-PCR. peptidylprolyl isomerase A (PPIA) is used as reference gene |
| Sterility Test | Incubation in CASO-Bouillion (Heipha) |
| Integrity of Expression Cassette in Viral Genomic DNA | PCR product is separated on a 1,3% agarose gel and stained with gel red. Only a single sharp band of the size of the analyzed sequence should be detected. |
Table 2. Optional Quality Control methods available upon customer request
| OPTIONAL QC METHODS | |
| Mycoplasma Test | PCR-based Mycoplasma Test Kit I/C from PromoCell |
| Endotoxin Measurement | Endosafe-nexgen-PTS Spectrometer from Charles River Laboratories. |
| SDS Page | Protein present in the final product is separated on a 10% SDS PAGE using Pierce Silver stain kit or SYBRO staining for visualization of proteins. |
| Functional Testing In Customer Defined Cells | Antibiotic selection, fluorescence detection, luciferase measurement. |
Scales and Timelines
SIRION offers several production stages for all levels of preclinical application – from cloning, vector development and manufacture Tables 3 & 4) to simple amplification of existing vector stock (Table 5). Production parameters can be adapted to specific projects – contact us for more information!
Table 3. Scales and timelines for cloning.
| Cloning | Timeline |
| Custom Construct Cloning (Gateway-based or classical) | 1-2 weeks |
Table 4. Scales and timelines for adenovirus production. (Recombination/first rescue based on cloned Ad5 shuttle plasmid, virus production, titration & QC.)
|
ADENOVIRUS PRODUCTION |
||
|
Service Type |
Scale |
Timelines |
|
Standard |
>1E9 IU |
3-5 weeks |
|
Medium |
>1E10 IU |
4-7 weeks |
|
AVVector Productivity Evaluation (for assessment of manufacturing conditions, timelines and costs) |
3-5 weeks |
|
|
Large |
>1E11 IU |
7-8 weeks |
|
X-Large |
>3E11 IU |
8-10 weeks |
Table 5. Scales and timelines for adenovirus amplification. (Amplification of an adenoviral vector stock, titration, & QC)
|
ADENOVIRUS AMPLIFICATION |
||
|
Service Type |
Scale |
Timelines |
|
Standard |
>1E9 IU |
2-3 weeks |
|
Medium |
>1E10 IU |
3-4 weeks |
|
Large |
>1E11 IU |
5-7 weeks |
|
X-Large |
>3E11 IU |
7-9 weeks |
